目前主要采用的化石能源开始出现耗竭,还产生了严重的环境污染与温室气体排放问题,寻找和利用新型绿色可再生能源成为一项重要任务。生物质及生物质基能源产物因其绿色、可再生、易获取的特点,愈发受到研究者的关注[1]。在众多的生物质基能源产物中,5-羟甲基糠醛(5-hydroxymethylfurfural,HMF)是一种重要的平台化合物,其具有呋喃环、羟基等活性基团,可以转化为5-乙氧基甲基糠醛(EMF)、乙酰丙酸乙酯(ethyl levulinate,EL)、2,5-呋喃二甲酸(furandicarboxylic acid,FDCA)、2,5-双(羟甲基)呋喃(2,5-bis(hydroxylmethyl)furan,BHMF)以及γ-戊内酯(γ-valerolactone,GVL)[2]等高附加值化学品。 其中,EMF可以由HMF同乙醇进行醚化反应直接制备获得。EMF能量密度为8.7 (kW·h)/L,同普通汽油的8.8 (kW·h)/L相近[3],比乙醇(6.1 (kW·h)/L)要高,EMF以质量比17∶83与柴油混溶时,其颗粒排放物、氮氧化物及硫氧化物排放量远远低于石化柴油,被认为是一种潜在的燃料或燃料添加剂[4]。本文对近年来国内外合成EMF的文献进行综述,对不同原料制取EMF的研究现状进行分析和讨论,并在此基础上对生物质制备EMF的研究趋势进行展望。
其中,EMF可以由HMF同乙醇进行醚化反应直接制备获得。EMF能量密度为8.7 (kW·h)/L,同普通汽油的8.8 (kW·h)/L相近[3],比乙醇(6.1 (kW·h)/L)要高,EMF以质量比17∶83与柴油混溶时,其颗粒排放物、氮氧化物及硫氧化物排放量远远低于石化柴油,被认为是一种潜在的燃料或燃料添加剂[4]。本文对近年来国内外合成EMF的文献进行综述,对不同原料制取EMF的研究现状进行分析和讨论,并在此基础上对生物质制备EMF的研究趋势进行展望。
1 制备原料
5-乙氧基甲基糠醛(5-hydroxymethylfurfural,EMF)可由糖类或木质纤维素类生物质直接转化制备,经历水解、脱水、醚化等反应过程。果糖和葡萄糖是制备EMF的2种主要单糖,当以果糖为原料时,EMF产率相对较高。因为果糖在酸性催化条件下,可以直接脱水生成HMF,再进一步醚化生成EMF。而以葡萄糖为原料时,葡萄糖要先异构化成果糖,再进行后续反应生成EMF,因此葡萄糖的EMF产率相较偏低[5]。表1为近些年以果糖、葡萄糖为原料制备EMF的研究情况。由表1可知,当以果糖为原料制取EMF时,反应温度通常为70~140 ℃,反应时间一般为10 min~24 h,其产率为60%以上,最高达到81%[6];而以葡萄糖为原料时,反应温度为120~160 ℃ ,反应时间一般为19 min~48 h,EMF产率最高为48%[7],一般为40%左右[8]。
当以纤维素类生物质为原料制备EMF时,其催化转化途径通常有2种,一是将生物质转化为 5-氯甲基糠醛(5-chloromethylfurfural,CMF)、5-溴甲基糠醛(5-bromomethyfurfural,BMF)等呋喃衍生物,然后进一步转化为EMF(图1)。Bredihhin等[21]以纤维素类生物质为原料,在1,2-二氯乙烷(1,2-dichloroethane)有机溶剂中,添加氢溴酸或者盐酸提供卤素,分别催化转化纤维素生成BMF和CMF,随后在乙醇中生成EMF,质量产率达到40%。值得一提的是,第二步反应中会有HBr或HCl生成,对环境不友好。二是利用木质纤维素和乙醇在酸催化作用下直接一锅法生成EMF。Chen等[22]以玉米秸秆为原料,在乙醇/四氢呋喃两相溶剂中一锅法制备EMF,反应经过2.9 h,产率达21.8%。直接催化转化途径工艺相对简单,反应时间较短,中间产物无需提纯与分离,但是产率相对较低。
表1 以果糖、葡萄糖为底物制备EMF的研究
Table 1 Studies on the preparation of EMF using fructose and glucose as substrates
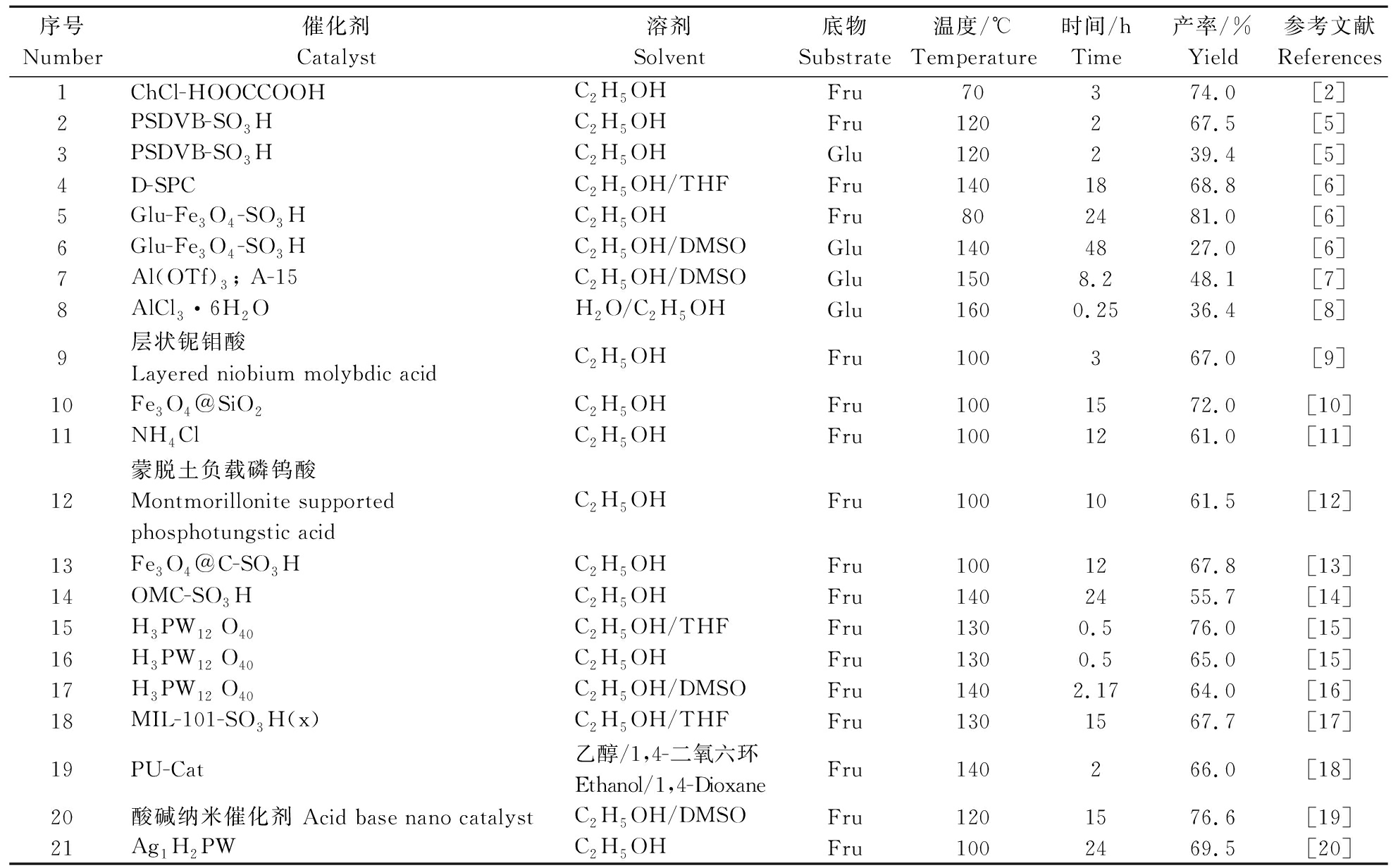
序号Number催化剂Catalyst溶剂Solvent底物Substrate温度/℃Temperature时间/hTime产率/%Yield参考文献References1ChCl-HOOCCOOHC2H5OHFru70374.0[2]2PSDVB-SO3HC2H5OHFru120267.5[5]3 PSDVB-SO3HC2H5OHGlu120239.4[5]4D-SPCC2H5OH/THFFru1401868.8[6]5Glu-Fe3O4-SO3HC2H5OHFru802481.0[6]6 Glu-Fe3O4-SO3HC2H5OH/DMSOGlu1404827.0[6]7Al(OTf)3; A-15C2H5OH/DMSOGlu1508.248.1[7]8AlCl3·6H2OH2O/C2H5OHGlu1600.2536.4[8]9层状铌钼酸Layered niobium molybdic acidC2H5OHFru100367.0[9]10Fe3O4@SiO2C2H5OHFru1001572.0[10]11NH4ClC2H5OHFru1001261.0[11]12蒙脱土负载磷钨酸Montmorillonite supportedphosphotungstic acidC2H5OHFru1001061.5[12]13Fe3O4@C-SO3HC2H5OHFru1001267.8[13]14OMC-SO3HC2H5OHFru1402455.7[14]15H3PW12 O40C2H5OH/THFFru1300.576.0[15]16H3PW12 O40C2H5OHFru1300.565.0[15]17H3PW12 O40C2H5OH/DMSOFru1402.1764.0[16]18MIL-101-SO3H(x)C2H5OH/THFFru1301567.7[17]19PU-Cat乙醇/1,4-二氧六环Ethanol/1,4-DioxaneFru140266.0[18]20酸碱纳米催化剂 Acid base nano catalystC2H5OH/DMSOFru1201576.6[19]21Ag1H2PWC2H5OHFru1002469.5[20]
注 Note:DMSO:二甲基亚砜 Dimethyl sulfoxide; THF:四氢呋喃 Tetrahydrofuran; Fru:果糖 Fructose; Glu:葡萄糖 Glucose.下表同 The same as follow Tables.

图1 纤维素催化转化制取EMF的可能途径
Fig.1 A possible pathway for the preparation of EMF from cellulose conversion
2 催化体系
以碳水化合物为原料制备EMF,通常需要在酸性催化剂作用下进行,根据催化剂在溶剂体系中的分布状态,大致可分为均相催化体系和非均相催化体系2种。
2.1 均相催化体系
液体酸是最为典型的一种均相催化剂,因离子在溶剂中分布均匀而具有较高的催化活性。液体酸可分为布朗斯台德酸 (Bronsted acid,简称B酸)和路易斯酸(Lewis acid,简称L酸),在EMF制备过程中,B酸对果糖的催化效果较好,对葡萄糖的异构化效果较差[23]。表2是近些年来以均相酸催化剂制备EMF的研究。从表2可以看出,在均相酸催化条件下,70~210 ℃下反应10 min~18 h, EMF产率最高可以达到92.9%[24]。Liu等[25]以硫酸为催化剂,在乙醇中以70 ℃反应18 h,HMF完全转化(转化率达100%),EMF产率达到79.8%。李凯[26]以较低浓度(0.1 mol/L)的硫酸作为催化剂,在乙醇中催化转化果糖、葡萄糖以及纤维素,产率分别为79.3%、21.8%、14.4%。
表2 均相酸催化剂制备EMF研究
Table 2 Studies on the preparation of EMF by homogeneous acid catalysts
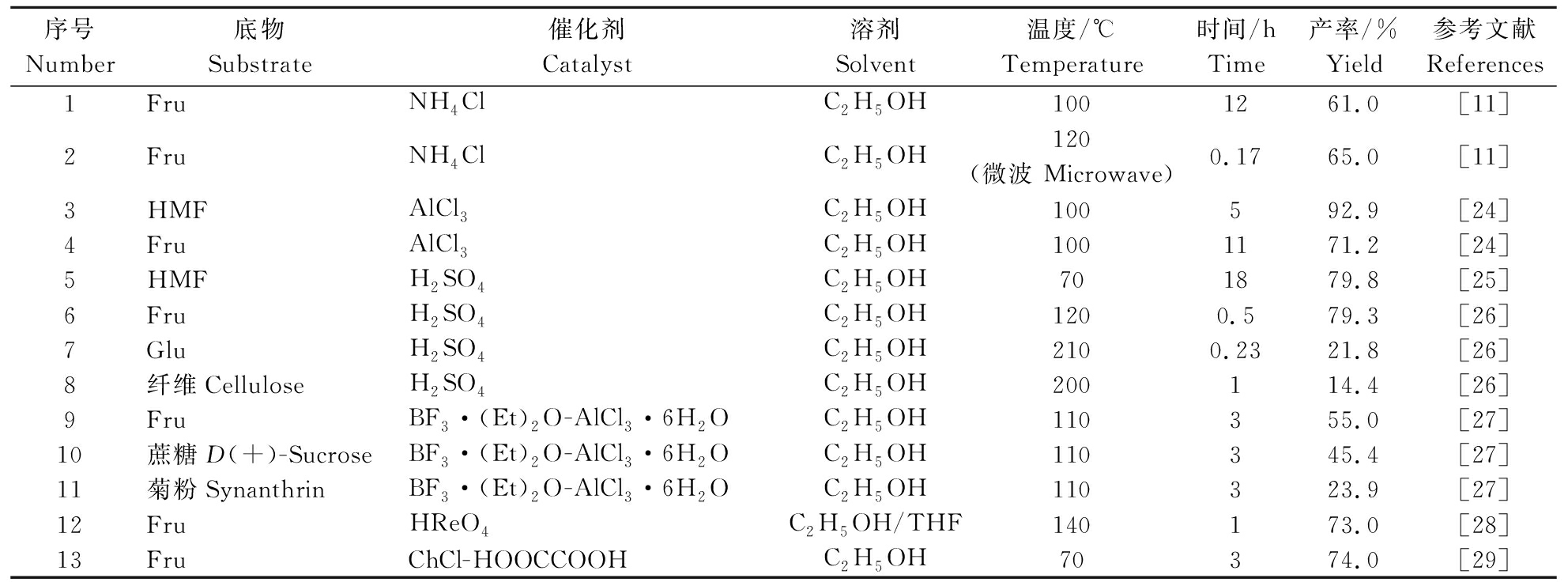
序号Number底物Substrate催化剂Catalyst溶剂Solvent温度/℃Temperature时间/hTime产率/%Yield参考文献References1FruNH4ClC2H5OH1001261.0[11]2FruNH4ClC2H5OH120(微波 Microwave)0.1765.0[11]3HMFAlCl3C2H5OH100592.9[24]4FruAlCl3C2H5OH1001171.2[24]5HMFH2SO4C2H5OH701879.8[25]6FruH2SO4C2H5OH1200.579.3[26]7GluH2SO4C2H5OH2100.2321.8[26]8纤维CelluloseH2SO4C2H5OH200114.4[26]9FruBF3·(Et)2O-AlCl3·6H2OC2H5OH110355.0[27]10蔗糖D(+)-SucroseBF3·(Et)2O-AlCl3·6H2OC2H5OH110345.4[27]11菊粉SynanthrinBF3·(Et)2O-AlCl3·6H2OC2H5OH110323.9[27]12FruHReO4C2H5OH/THF140173.0[28]13FruChCl-HOOCCOOHC2H5OH70374.0[29]
注 Note:HMF:5-羟甲基糠醛 5-Hydroxymethylfurfural.
路易斯酸能够促进葡萄糖的异构化,因而催化葡萄糖类原料醇解转化制备EMF通常需要路易斯酸的参与[27]。Liu等[24]利用AlCl3为催化剂,催化果糖制取EMF,在100 ℃下反应11 h,产率为71.2%。反应液经过蒸发萃取等过程,回收得到的催化剂AlCl3活性基本不变,具有良好的可重复性。Liu等[11]利用NH4Cl作为催化剂,一锅法催化果糖制取EMF,在乙醇溶剂中分别采用油浴以及微波加热,最终EMF产率分别为61.0%、65.0%。
离子液是一种低温或常温下呈现液态的盐,也称为低温熔融盐。酸性离子液在部分反应中可起到催化和萃取的功能,具有挥发性低、毒性低、极性高、酸度可调、容易分离回收、催化效率高等优点[30],被认为是一种绿色的催化体系。Guo等[31]以果糖作为原料,以酸性离子液体[C4MIM][HSO4]-作为共溶剂以及催化剂,在乙醇溶剂体系中120 ℃下反应20 min,得到了83%的EMF。离子液体在重复利用5次后,对EMF产率仅有轻微影响。Alam等[32]采用磺酸功能化离子液体[DMA][CH3SO3]为催化剂,以野生蘑菇为原料,在乙醇溶剂中一锅法进行反应制取液体燃料,在120 ℃下反应20 h后,得到产率为28%的液体燃料(EMF和EL物质的量之比为9∶1),离子液体经过回收利用后,再进行重复实验,3次重复利用后催化活性只有轻微下降,说明其重复回收实验可行。尽管离子液体催化体系因其优异的催化性能而受到广泛的关注,但离子液体的成本问题函待解决,开发成本低廉、制备简便且EMF选择性更高的新型离子液体催化剂将成为进一步的研究方向。
2.2 非均相催化体系
固体酸具有易与反应体系分离、不腐蚀设备、后处理简单、热稳定性强等优势,是目前研究的重点。表3是近些年固体酸催化制备EMF的研究。由表3可知,在80~150 ℃下反应30 min~24 h,最高可以通过固体酸催化得到91.5%的EMF。生物质制备EMF用到的固体酸主要有分子筛类、磺酸功能化催化剂、杂多酸类、离子交换树脂类催化剂。其中,分子筛类催化剂是具有网状结构的天然或人工合成的化学物质[33],其骨架由SiO4和AlO4通过顶点按三维结果堆积而成。分子筛目前广泛应用于HMF、果糖、葡萄糖等为底物的EMF制备实验。Che等[41]制备了H4SiW12O40/MCM-41纳米微粒催化剂,在乙醇催化体系中,催化HMF醚化制取EMF,反应在90 ℃下进行4 h,EMF产率为77.2%。伴随着其活性的轻微降低,催化剂可重复利用5次。
表3 非均相酸催化剂制备EMF研究
Table 3 Studies on the preparation of EMF by Heterogeneous acid catalyst
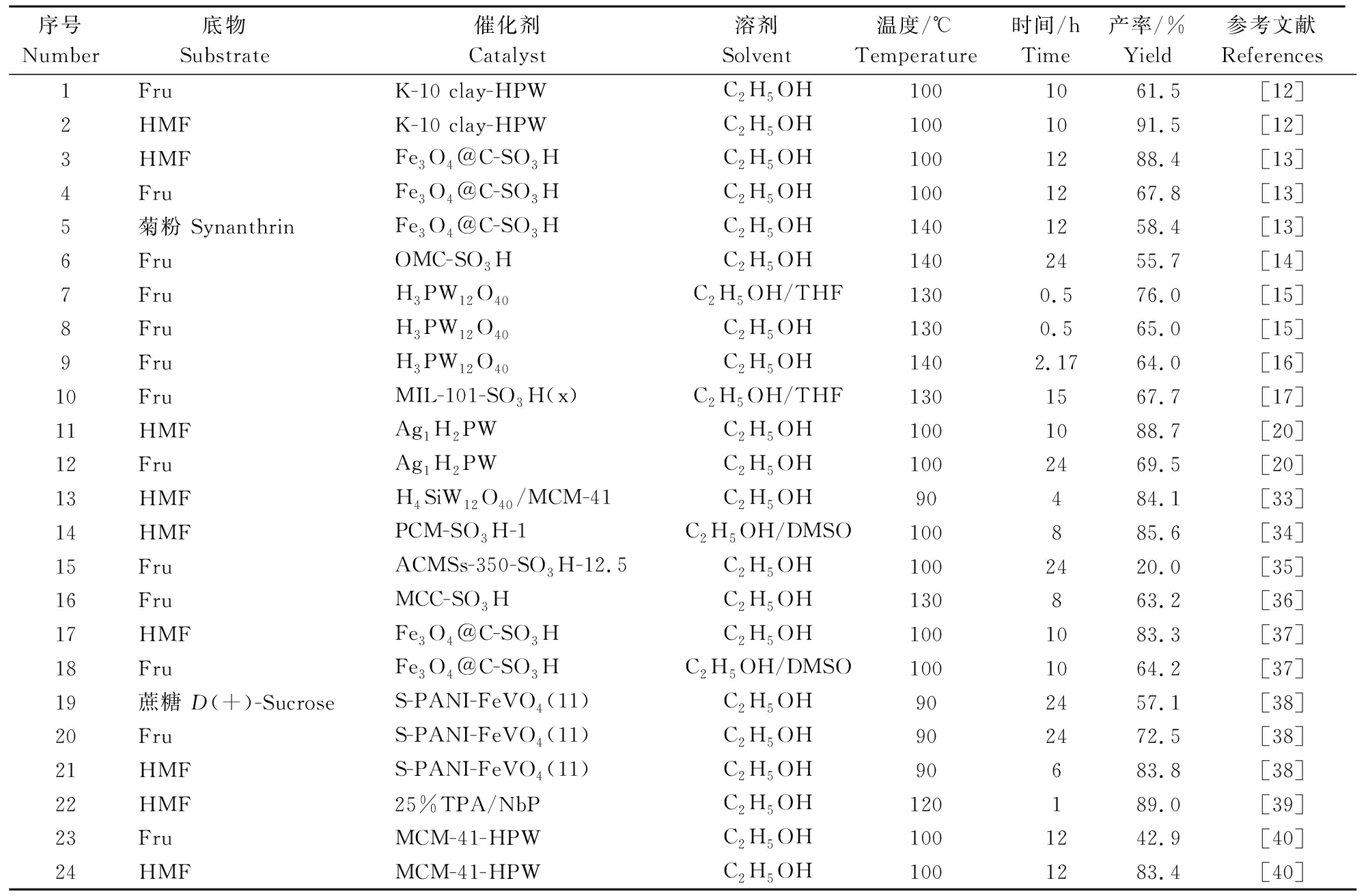
序号Number底物Substrate催化剂Catalyst溶剂Solvent温度/℃Temperature时间/hTime产率/%Yield参考文献References1FruK-10 clay-HPWC2H5OH1001061.5[12]2HMFK-10 clay-HPWC2H5OH1001091.5[12]3HMFFe3O4@C-SO3HC2H5OH1001288.4[13]4FruFe3O4@C-SO3HC2H5OH1001267.8[13]5菊粉 SynanthrinFe3O4@C-SO3HC2H5OH1401258.4[13]6FruOMC-SO3HC2H5OH1402455.7[14]7FruH3PW12O40C2H5OH/THF1300.576.0[15]8FruH3PW12O40C2H5OH1300.565.0[15]9FruH3PW12O40C2H5OH1402.1764.0[16]10FruMIL-101-SO3H(x)C2H5OH/THF1301567.7[17]11HMFAg1H2PWC2H5OH1001088.7[20]12FruAg1H2PWC2H5OH1002469.5[20]13HMFH4SiW12O40/MCM-41C2H5OH90484.1[33]14HMFPCM-SO3H-1C2H5OH/DMSO100885.6[34]15FruACMSs-350-SO3H-12.5C2H5OH1002420.0[35]16FruMCC-SO3HC2H5OH130863.2[36]17HMFFe3O4@C-SO3HC2H5OH1001083.3[37]18FruFe3O4@C-SO3HC2H5OH/DMSO1001064.2[37]19蔗糖 D(+)-SucroseS-PANI-FeVO4(11)C2H5OH902457.1[38]20FruS-PANI-FeVO4(11)C2H5OH902472.5[38]21HMFS-PANI-FeVO4(11)C2H5OH90683.8[38]22HMF25%TPA/NbPC2H5OH120189.0[39]23FruMCM-41-HPWC2H5OH1001242.9[40]24HMFMCM-41-HPWC2H5OH1001283.4[40]
磺酸功能化催化剂有较高催化性能,易分离回收,绿色环保[33],催化剂活性可由负载酸量来调节。磺酸功能化催化剂广泛运用于HMF、果糖、葡萄糖、菊粉等原料的催化制备上,分别可以制备得到约91.5%、67%、39%、85%的EMF。Liu等[17]合成了一系列MIL-101-SO3H(x)高分子磺化材料,在乙醇、四氢呋喃两相溶剂中反应15 h,反应温度为130 ℃,催化果糖得到产率为67.7%的EMF。相较于非生物质基固体催化剂,生物质基磺化固体酸催化剂具有更好的生物亲和力和更低的制备成本[42],比如以碳化生物质为原料,通过磺化法可轻易制备生物质固体酸催化剂。姚远[34]以自制的磺化磁性碳基固体酸PCM-SO3H-1为催化剂,在乙醇和二甲基亚砜的两相有机溶剂体系中一锅法催化HMF制取EMF,在100 ℃的反应温度下反应8 h,EMF产率可得85.6%。在反应结束后,催化剂可在外加磁场作用下,与反应混合物轻易分离并回收,经多次重复使用,催化剂仍可保持较高活性。
杂多酸是一种无机酸,含有由不同种类的含氧酸根阴离子缩合而成的称为杂多阴离子(如WO42- +PO43-→PW12O403-)作为其酸根[43],通常用于HMF以及果糖的催化转化。Yang等[15]直接利用磷钨酸H3PW12O40为催化剂,在乙醇溶剂中一锅法催化果糖制取EMF,反应采用微波加热,在130 ℃下反应30 min,得到65%的EMF。Wang等[16]以磷钨酸作为催化剂,在140 ℃下催化果糖制取EMF,反应进行130 min,可得到64%的EMF。
酸性离子交换树脂是一种催化活性高且具有较高的化学稳定性以及较高机械强度的高分子化合物,含有磺酸基、羧基等官能团[44],常用于酯交换反应,在催化生物质制备EMF中同样得到应用。Zuo等[35]以酸性离子交换树脂Amberlyst-15为催化剂,分别以果糖、菊粉为原料制备EMF,得到了77.3%、65.2%的EMF。随后以CrCl3作为改性物质,对Amberlyst-15进行改性,并以此改性催化剂催化葡萄糖、蔗糖制备EMF,产率分别为46.7%、50.2%;且改性前后的离子交换树脂均具有良好的重复使用性质,数次重复使用,实验中没有明显的活性丧失。Dai等[45]自制了固体有机磺化聚合催化剂D-SPC,在乙醇与四氢呋喃两相溶剂中一锅法反应制取EMF,产率可达68.8%;并与多种催化剂进行对比,其中Amberlyst-15催化剂在同等反应条件下,EMF产率可达52.9%。Yu等[7]使用铝基催化剂Al(OTf)3与Amberlyst-15树脂协同催化葡萄糖催化转化制备EMF,反应以体积比1∶1的乙醇、DMSO为溶剂体系,在150 ℃的反应温度下连续进行8.2 h,制备得到了48.1%的EMF。
3 溶剂体系
3.1 乙醇体系
乙醇是制备EMF最常见的溶剂,不仅来源广泛、成本低廉、无污染、易于回收,乙醇还是一锅法制取EMF工艺中必要的反应物。在目前EMF制取研究中,很多研究者采用乙醇作为反应物和反应溶剂[45],在乙醇中直接进行HMF的醚化反应,产率最高可达92.9%。此外,乙醇在碳水化合物的转化中还可以抑制腐殖质的生成[46]。Liu等[24]在乙醇中催化转化HMF制备EMF,以AlCl3作为催化剂,在100 ℃下得到92.9%的EMF,将果糖作为原料,EMF产率也可达61.8%;张泽会等[10]在乙醇参与下,以自制磁性纳米粒子负载磺酸的固体酸催化剂催化果糖合成EMF,产率高达72.3%。而以菊粉作为底物,收率同样可以达到63.3%。乙醇作为溶剂简单易获取、无毒、环保、易于回收以及分离,是备受关注的一种绿色溶剂,也是目前使用最为广泛的溶剂体系。
3.2 两相溶剂体系
溶剂体系中加入共溶剂,组成两相溶剂体系用于催化转化生物质制备EMF,是一种提升目标产物产率的常见方法。通常以某种有机溶剂或者极性溶剂作为乙醇的共溶剂,用于EMF的催化转化,常见的共溶剂有DMSO、THF、1,4-二氧六环等。在同样或类似的反应条件下,共溶剂的加入通常可以显著提升产率。表4为共溶剂在EMF制备中的应用。由表4可知,在两相溶剂体系内,底物在100~150 ℃下反应0.5~24 h,最高可得到89.5%的EMF[12]。
表4 两相溶剂体系在EMF制备中的研究
Table 4 Study of two-phase solvent system in EMF preparation
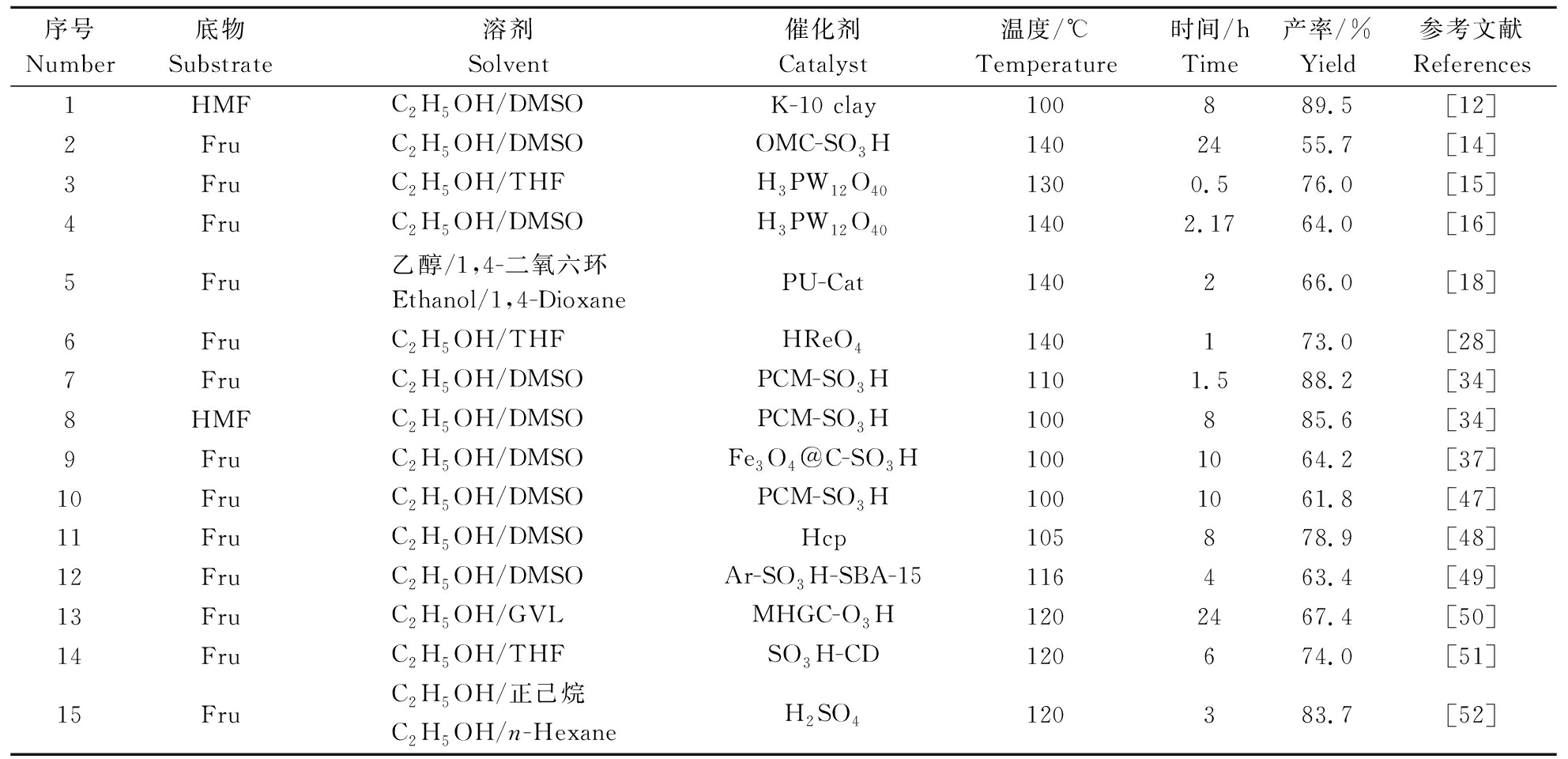
序号Number底物Substrate溶剂Solvent催化剂Catalyst温度/℃Temperature时间/hTime产率/%Yield参考文献References1HMFC2H5OH/DMSOK-10 clay100889.5[12] 2FruC2H5OH/DMSOOMC-SO3H1402455.7[14]3FruC2H5OH/THFH3PW12O401300.576.0[15]4FruC2H5OH/DMSOH3PW12O401402.1764.0[16]5Fru乙醇/1,4-二氧六环Ethanol/1,4-DioxanePU-Cat140266.0[18]6FruC2H5OH/THFHReO4140173.0[28]7FruC2H5OH/DMSOPCM-SO3H1101.588.2[34]8HMFC2H5OH/DMSOPCM-SO3H100885.6[34]9FruC2H5OH/DMSOFe3O4@C-SO3H1001064.2[37]10FruC2H5OH/DMSOPCM-SO3H1001061.8[47]11FruC2H5OH/DMSOHcp105878.9[48]12FruC2H5OH/DMSOAr-SO3H-SBA-15116463.4[49]13FruC2H5OH/GVLMHGC-O3H 1202467.4[50]14FruC2H5OH/THFSO3H-CD120674.0[51]15FruC2H5OH/正己烷C2H5OH/n-HexaneH2SO4120383.7[52]
目前,制备EMF中使用到的共溶剂有二甲亚砜(DMSO)、四氢呋喃(THF)、γ-戊内酯等,这些共溶剂都可以在一定程度上提高EMF的产率。DMSO具有优良的热稳定性、高极性、高沸点等特性,能溶于水和乙醇等有机物,通常用作极性和渗透性有机溶剂被广泛用于化学、医药、电子和金属冶炼[53],同时也作为共溶剂被广泛应用于EMF的制备之中。研究表明,DMSO的加入可以促进EMF的生成,并减少副产物的形成[49]。Zhang等[48]制备了一种新型磺酸改性有机超交联聚合物催化剂,并在乙醇中加入5 mL DMSO作为反应溶剂体系,在最佳反应条件下,EMF产率提高了18%,达到78.9%。Morales等[49]研究了二甲基亚砜与乙醇作为共溶体系时,果糖醇解制备EMF的催化转化规律,二甲基亚砜体积分数8.3%的条件下,在116 ℃下反应4 h,可以得到EMF的最优产率,为63.4%。尽管多方面研究表明,DMSO是一种可以促进EMF高效率转化的有机溶剂,但由于其本身存在弱毒性,故寻找更加绿色、安全且环保的溶剂,仍是研究热点。
THF可有效提高EMF产率并抑制副反应产生果糖转化为EMF的中间体产物HMF[54]。Yang等[15]直接利用磷钨酸H3PW12O40为催化剂,一锅法催化果糖制取EMF,最佳反应条件下可得到产率为65%的EMF。而当改变溶剂体系为乙醇、四氢呋喃(V/V=5∶3)共溶剂体系时,可以使EMF产率提高12%。同DMSO类似,THF具有弱毒性,不够安全环保是其严重缺陷。
Li等[18]在140 ℃的乙醇/1,4-二氧六环(V/V=70∶30)两相溶剂中,以自制聚氨酯基催化剂将果糖一锅转化为EMF,并获得66.0%的EMF收率。在同等反应条件下,乙醇溶剂作为对照组,EMF收率仅有56.2%。
Bai等[50]进行了在γ-戊内酯与乙醇组成的共溶系统中,制备磁性碳基固体酸催化剂用于催化果糖制备EMF的研究。研究发现,加入γ-戊内酯可以明显提升EMF产率。相比纯的乙醇溶剂,γ-戊内酯可以提供疏水环境,并减少腐殖质的生成。在最优的反应条件下,两相溶剂体系EMF产率可以达到67.4%。Xu等[52]研究了正己烷作为共溶剂对于催化碳水化合物一锅法制取EMF和EL的影响,在最佳反应温度和反应时间下,两相溶剂体系可以提升5.73%的EMF和EL的总产率。两相溶剂体系虽然能提高产物收率,但也存在产品不易与溶剂分离、成本较高、回收率较低、对环境污染等问题。因此,开发绿色环保的新型溶剂并提高其回收利用率将成为未来的研究方向。
4 结语与展望
EMF是具有良好燃烧性能的替代燃料和化学品,目前,研究人员对生物质制备EMF进行了大量的研究,虽然果糖类原料可以获得较高的EMF产率,但是葡萄糖类和纤维素类原料的EMF转化产率较低。固体催化剂的使用可以实现催化剂的重复利用,简化产物的分离提取工艺。共溶剂的使用能够有效提高反应转化率,但是溶剂的毒性、分离和重复利用依然是制约EMF产业化的主要原因。由于糖类具有较高的生产成本,未来的原料应该是葡萄糖类的纤维素类生物质原料,若能实现生物质到EMF的大规模转化,将极大地缓解能源压力,经济与环境问题也会在一定程度上得到解决。因此,开发高效、绿色、可回收利用的固体催化剂以及与之相匹配的无毒、高效的两相溶剂体系依然是未来的主要研究内容。
EMF作为汽油和柴油添加剂,其发展要依附于石油化工,而石油行业正面临着风力发电、太阳能和生物质能等新能源产业的冲击。近期油价暴跌,这对我们来说是挑战,同样是机遇。油价降低会一定程度上突显EMF制备成本偏高的劣势,这是挑战;但是低油价可以将普通消费者的目光从新能源转移到石油化工上,石油的应用将带动石油相关产品的发展。
[1] 徐桂转,张百良.生物柴油的研究与进展[J].华中农业大学学报,2005,24(6):644-650.XU G Z,ZHANG B L.Research and progress of biodiesel[J].Journal of Huazhong Agricultural University,2005,24(6):644-650(in Chinese with English abstract).
[2] GAWADE A B,YADAV G D.Microwave assisted synthesis of 5-ethoxymethylfurfural in one pot from D-fructose by using deep eutectic solvent as catalyst under mild condition[J].Biomass and bioenergy,2018,117:38-43.
[3] 邓京波.中国开发出可将碳水化合物转化为液体生物燃料的新型催化剂[J].石油炼制与化工,2015,46(6):56.DENG J B.China has developed a new catalyst that can convert carbohydrates into liquid biofuels[J].Petroleum processing and petrochemicals,2015,46(6):56(in Chinese with English abstract).
[4] 辛浩升.催化转化葡萄糖制备高附加值化学品5-羟甲基糠醛和5-乙氧基甲基糠醛的研究[D].合肥:安徽建筑大学,2018.XIN H S.Study on catalytic conversion of glucose to prepare high value chemicals 5-hydroxymethylfurfural and 5-ethoxymethylfurfural[D].Hefei:Anhui Jianzhu University,2018(in Chinese with English abstract).
[5] ZHANG L X,ZHU Y J,TIAN L,et al.One-pot alcoholysis of carbohydrates to biofuel 5-ethoxymethylfufural and 5-methoxymethylfufural via a sulfonic porous polymer[J].Fuel processing technology,2019,193:39-47.
[6] THOMBAL R S,JADHAV V H.Application of glucose derived magnetic solid acid for etherification of 5-HMF to 5-EMF,dehydration of sorbitol to isosorbide,and esterification of fatty acids[J].Tetrahedron letters,2016,57(39):4398-4400.
[7] YU X,GAO X Y,PENG L C,et al.Intensified 5-ethoxymethylfurfural production from biomass components over aluminum-based mixed-acid catalyst in co-solvent medium[J].Chemistry select,2018,3(47):13391-13399.
[8] YANG Y,HU C W,ABU-OMAR M M.Conversion of glucose into furans in the presence of AlCl3 in an ethanol-water solvent system[J].Bioresource technology,2012,116:190-194.
[9] YANG F,TANG J J,OU R,et al.Fully catalytic upgrading synthesis of 5-ethoxymethyl furfural from biomass-derived 5-hydroxymethylfurfural over recyclable layered-niobium-molybdate solid acid[J/OL].Applied catalysis B:environmental,2019,256:117786.[2019-12-20].https://doi.org/10.1016/j.apcatb.2019.117786.
[10] 张泽会,刘冰,肖少华.磁性固体酸催化碳水化合物“一锅法”制备生物燃料5-乙氧基甲基糠醛[C]//中国化学会催化委员会.第十四届全国青年催化学术会议论文集.长春:中国化学会,2013:1-3.ZHANG Z H,LIU B,XIAO S H.One-pot synthesis of 5-ethoxymethylfurfural from carbohydrates catalyzed by magnetic solid acid[C]//The Catalysis Society of China.Proceedings of the 14th national catalysis conference.Changchun:Chinese Chemical Society,2013:1-3(in Chinese with English abstract).
[11] LIU J T,TANG Y,WU K G,et al.Conversion of fructose into 5-hydroxymethylfurfural (HMF) and its derivatives promoted by inorganic salt in alcohol[J].Carbohydrate research,2012,350:20-24.
[12] LIU A Q,LIU B,WANG Y M,et al.Efficient one-pot synthesis of 5-ethoxymethylfurfural from fructose catalyzed by heteropolyacid supported on K-10 clay[J].Fuel,2014,117:68-73.
[13] YUAN Z L,ZHANG Z H,ZHENG J D,et al.Efficient synthesis of promising liquid fuels 5-ethoxymethylfurfural from carbohydrates[J].Fuel,2015,150:236-242.
[14] WANG J M,ZHANG Z H,JIN S W,et al.Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sufonic acid-functionalized mesoporous carbon catalyst[J].Fuel,2017,192:102-107.
[15] YANG Y,ABU-OMAR M M,HU C W.Heteropolyacid catalyzed conversion of fructose,sucrose,and inulin to 5-ethoxymethylfurfural,a liquid biofuel candidate[J].Applied energy,2012,99(2):80-84.
[16] WANG H L,DENG T S,WANG Y X,et al.Efficient catalytic system for the conversion of fructose into 5-ethoxymethylfurfural[J].Bioresour Technol,2013,136(5):394-400.
[17] LIU X F,LI H,PAN H,et al.Efficient catalytic conversion of carbohydrates into 5-ethoxymethylfurfural over MIL-101-based sulfated porous coordination polymers[J].Journal of energy chemistry,2016,25(3):523-530.
[18] LI J L,WANG Y Q,LU B Q,et al.Protonic acid catalysis of sulfonated carbon material:tunable and selective conversion of fructose in low-boiling point solvent[J].Applied catalysis A:general,2018,566:140-145.
[19] LI H,GOVIND K S,KOTNI R,et al.Direct catalytic transformation of carbohydrates into 5-ethoxymethylfurfural with acid-base bifunctional hybrid nanospheres[J].Energy conversion and management,2014,88:1245-1251.
[20] REN Y S,LIU B,ZHANG Z H,et al.Silver-exchanged heteropolyacid catalyst (Ag1H2PW):an efficient heterogeneous catalyst for the synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose[J].Journal of industrial and engineering chemistry,2015,21:1127-1131.
[21] BREDIHHIN A,MAEORG U,VARES L.Evaluation of carbohydrates and lignocellulosic biomass from different wood species as raw material for the synthesis of 5-bromomethyfurfural[J].Carbohydrate research,2013,375:63-67.
[22] CHEN B L,XU G Z,ZHENG Z B,et al.Efficient conversion of corn stover into 5-ethoxymethylfurfural catalyzed by zeolite USY in ethanol/THF medium[J].Industrial crops and products,2019,129:503-511.
[23] CHEN B L,YAN G H,CHEN G F,et al.Recent progress in the development of advanced biofuel 5-ethoxymethylfurfural[J].BMC energy,2020,2(1):1-13.
[24] LIU B,ZHANG Z H,HUANG K C,et al.Efficient conversion of carbohydrates into 5-ethoxymethylfurfural in ethanol catalyzed by AlCl3[J].Fuel,2013,113:625-631.
[25] LIU B,ZHANG Z H,DENG K J.Efficient one-pot synthesis of 5-(ethoxymethyl)furfural from fructose catalyzed by a novel solid catalyst[J].Industrial & engineering chemistry research,2012,51(47):15331-15336.
[26] 李凯.一锅法催化生物质制取5-乙氧基甲基糠醛试验研究[D].郑州:河南农业大学,2016.LI K.One-pot synthesis of 5-ethoxymethlfurfural from carbohydrate catalyzed[D].Zhengzhou:Henan Agricultural University,2016(in Chinese with English abstract).
[27] JIA X Q,MA J P,CHE P H,et al.Direct conversion of fructose-based carbohydrates to 5-ethoxymethylfurfural catalyzed by AlCl3·6H2O/BF3·(Et)2O in ethanol[J].Journal of energy chemistry,2013,22(1):93-97.
[28] BERNARDO J R,OLIVEIRA M C,FERNANDES A C.HReO4 as highly efficient and selective catalyst for the conversion of carbohydrates into value added chemicals[J].Molecular catalysis,2019,465:87-94.
[29] GAWADE A B,YADAV G D.Microwave assisted synthesis of 5-ethoxymethylfurfural in one pot from D-fructose by using deep eutectic solvent as catalyst under mild condition[J].Biomass and bioenergy,2018,117:38-43.
[30] 齐玉欢,毕维强,王旭,等.离子液体催化氧化脱硫技术进展[J].石化技术与应用,2019,37(2):144-148.QI Y H,BI W Q,WANG X,et al.Progress in catalytic oxidation and desulfurization of ionic liquids technology[J].Petrochemical technology & application,2019,37(2):144-148(in Chinese with English abstract).
[31] GUO H X,QI X H,HIRAGA Y Y,et al.Efficient conversion of fructose into 5-ethoxymethylfurfural with hydrogen sulfate ionic liquids as co-solvent and catalyst[J].Chemical engineering journal,2017,314:508-514.
[32] ALAM M I,DE S,KHAN T S,et al.Acid functionalized ionic liquid catalyzed transformation of non-food biomass into platform chemical and fuel additive[J].Industrial crops and products,2018,123:629-637.
[33] 陈涛,彭林才.新型生物燃料5-乙氧基甲基糠醛的合成进展[J].化学通报,2017,81(1):45-51.CHEN T,PENG L C.Advances in the synthesis of novel biofuel 5-ethoxymethylfurfural[J].Chemistry bulletin,2017,81(1):45-51(in Chinese with English abstract).
[34] 姚远.磁性碳质材料催化糖类制备5-羟甲基糠醛及其衍生物的研究[D].无锡:江南大学,2016.YAO Y.The research on conversion of carbohydrates into 5-hydroxymethylfurfural and its derivative over magnetic carbonaceous material[D].Wuxi:Jiangnan University,2016(in Chinese with English abstract).
[35] ZUO M,LE K,FENG C Y,et al.An effective pathway for converting carbohydrates to biofuel 5-ethoxymethylfurfural via 5-hydroxymethylfurfural with deep eutectic solvents (DESs)[J].Industrial crops and products,2018,112:18-23.
[36] CHEN T,PENG L C,YU X,et al.Magnetically recyclable cellulose-derived carbonaceous solid acid catalyzed the biofuel 5-ethoxymethylfurfural synthesis from renewable carbohydrates[J].Fuel,2018,219:344-352.
[37] YAO Y,GU Z,WANG Y,et al.Magnetically-recoverable carbonaceous material:an efficient catalyst for the synthesis of 5-hydroxymethylfurfural and 5-ethoxymethylfurfural from carbohydrates[J].Russian journal of general chemistry,2016,86(7):1698-1704.
[38] KUMAR A,SRIVASTAVA R.FeVO4 decorated-SO3H functionalized polyaniline for direct conversion of sucrose to 2,5-diformylfuran & 5-ethoxymethylfurfural and selective oxidation reaction[J].Molecular catalysis,2019,465:68-79.
[39] KUMARI P K,RAO B S,LAKSHMI D D,et al.Tungstophosphoric acid supported on mesoporouus niobiumoxophosphate:an efficient solid acid catalyst for etherification of 5-hydroxymethylfurfural to 5-ethoxymethylfurfural[J].Catalysis today,2019,325:53-60.
[40] LIU A Q,ZHANG Z H,FANG Z F,et al.Synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose in ethanol catalyzed by MCM-41 supported phosphotungstic acid[J].Journal of industrial and engineering chemistry,2014,20(4):1977-1984.
[41] CHE P H,LU F,ZHANG J J,et al.Catalytic selective etherification of hydroxyl groups in 5-hydroxymethylfurfural over H4SiW12O40/MCM-41 nanospheres for liquid fuel production[J].Bioresource technology,2012,119:433-436.
[42] YUAN Z L,ZHANG Z H,ZHENG J D,et al.Efficient synthesis of promising liquid fuels 5-ethoxymethylfurfural from carbohydrates[J].Fuel,2015,150:236-242.
[43] 梁建军.多酸化学简史[J].大学化学,2007,22(1):67-70.LIANG J J.A brief history of polyacid chemistry[J].University chemistry,2007,22(1):67-70(in Chinese).
[44] 赵利飞,刘喜莹,李文红,等.Amberlyst-15型离子交换树脂催化γ-丁内酯酯交换反应的研究[J].化学研究与应用,2012,24(3):488-492.ZHAO L F,LIU X Y,LI W H,et al.Study on transesterification of γ-butyrolactone with alcohols catalyzed by amberlyst-15[J].Chemical research and application,2012,24(3):488-492(in Chinese with English abstract).
[45] DAI J H,LIU Z B,HU Y X,et al.Adjusting the acidity of sulfonated organocatalyst for the one-pot production of 5-ethoxymethylfurfural from fructose[J].Catalysis science & technology,2019,9(2):483-492.
[46] LUIGI D B,GEORGIA A,CAMILLA B,et al.Lewis-Bronsted acid catalysed ethanolysis of the organic fraction of municipal solid waste for efficient production of biofuels[J].Bioresource technology,2018,266:297-305.
[47] 姚远,王海军.磁性碳质材料催化糖类制备5-羟甲基糠醛和5-乙氧基甲基糠醛的研究[J].应用化工,2016(7):1258-1261.YAO Y,WANG H J.Magnetic carbonaceous solid acid catalyst for the synthesis of 5-hydroxymethylfurfural and 5-ethoxymethylfurfural from carbohydrates[J].Applied chemical industry,2016(7):1258-1261(in Chinese with English abstract).
[48] ZHANG J,DONG K J,LUO W M,et al.Catalytic upgrading of carbohydrates into 5-ethoxymethylfurfural using SO3H functionalized hyper-cross-linked polymer based carbonaceous materials[J].Fuel,2018,234:664-673.
[49] MORALES G,PANIAGUA M,MELERO J A,et al.Efficient production of 5-ethoxymethylfurfural from fructose by sulfonic mesostructured silica using DMSO as co-solvent[J].Catalysis today,2017,279:305-316.
[50] BAI Y Y,SU S H,WANG S Z,et al.Catalytic conversion of carbohydrates into 5-ethoxymethylfurfural by a magnetic solid acid using γ-valerolactone as a co-solvent[J].Energy technology,2018,6(10):1951-1958.
[51] MANEECHAKR P,KARNJANAKOM S.Selective conversion of fructose into 5-ethoxymethylfurfural over green catalyst[J].Research on chemical intermediates,2019,45(2):743-756.
[52] XU G Z,CHEN B L,ZHENG Z B,et al.One-pot ethanolysis of carbohydrates to promising biofuels:5-ethoxymethylfurfural and ethyl levulinate[J].Asia-Pacific journal of chemical engineering,2017,12(4):527-535.
[53] 辛姣,王凤,夏晓梅,等.以二甲基亚砜为溶剂分离多种共沸物系的研究[J].安徽化工,2018,44(6):22-23,26.XIN J,WANG F,XIA X M,et al.Study on the separation of multiple azeotropes with dimethyl sulfoxide as solvent[J].Anhui chemical industry,2018,44(6):22-23,26(in Chinese with English abstract).
[54] 刘晓芳.功能化金属有机框架的制备及其在催化生物质转化中的应用[D].贵阳:贵州大学,2017.LIU X F.Preparation of functional metal-organic framework and its application in catalytic biomass conversion[D].Guiyang:Guizhou University,2017(in Chinese with English abstract).