水牛是哺育中华民族的主要畜种之一。据联合国粮农组织(FAO)最新统计数据,2017年,我国水牛存栏总数2 347.2万头,居世界第三位,但一直作为役用动物饲养,其很多经济性状都没能得到充分的利用,因此被认为是最具有开发潜力的家畜[1]。水牛繁殖性能较弱,与黄牛相比,水牛原始卵泡储备量低,发情周期中各发育阶段总卵泡数也较少,卵泡闭锁发生率较高,卵母细胞和胚胎发育能力低[2]。提高水牛繁殖性能,特别是提高卵母细胞和早期胚胎发育潜力的研究工作极具挑战且意义重大。
卵母细胞质量决定胚胎质量,胚胎损失对黄牛和水牛等繁殖周期长、繁殖性能低的家畜养殖者意味着巨大的经济损失。在畜牧生产中,40%以上的胚胎损失发生在妊娠的 8~17 d,奶牛约75%~80%的繁殖损失是早期胚胎死亡引起的[3]。前列腺素作为一种重要的生殖激素,参与了哺乳动物输卵管运动与排卵、黄体功能调节、胚胎发育与着床、分娩与母性行为等重要的生殖活动,早期研究表明,前列腺素F2α(PGF2α)对部分哺乳动物体外胚胎[4-5]的发育有阻碍作用。研究发现,PGF2α受体PTGFR广泛存在于奶牛的生殖器官中[6],胚胎质量和怀孕率与子宫腔PGF2α浓度呈负相关[7]。笔者所在课题组前期研究证实,水牛卵母细胞中存在PGF2α受体(PTGFR)信号,因此推测抑制PGF2α信号有望成为一种提高水牛繁殖力的有效手段。本试验旨在通过探究抑制PGF2α受体(PTGFR)信号对提高水牛卵母细胞成熟和早期胚胎发育潜力的作用及机制,为在生产实践中减少妊娠早期损失、提高配种受胎率提供新思路。
1 材料与方法
1.1 材 料
水牛卵巢采自武汉市华强家禽批发中心。水牛宰杀后立即摘取卵巢,清洗其表面血迹后置于恒温37 ℃的 PBS缓冲液(1%双抗)中尽快送回实验室,再用PBS缓冲液清洗数次,放入25 ℃恒温水浴锅中待用;水牛冻精,购自湖北省畜禽育种中心;一抗PTGFR IgG购自北京博奥森公司;二抗IgG-FITC购自武汉博士德公司;AnnexinV-FITC细胞凋亡检测试剂盒购自凯基生物公司;AL-8810购自Cayman公司;FCS、TCM-199购自Gibco公司;其他均购自Sigma公司。
1.2 溶液的配制
1)捡卵液(CCM):3.331 6 g NaCl +0.119 3 g KCl+0.020 4 g NaH2PO4+0.7 mL乳酸钠+1.191 5 g HEPES+0.011 0 g丙酮酸钠+1.093 g山梨糖醇+0.084 g NaHCO3+0.012 5 g庆大霉素+0.05 g PVA+0.509 9 g MgCl2·6H2O+0.147 0 g CaCl2·2H2O,加双蒸水定容至500 mL,用NaOH溶液调节pH值为7.4,过滤灭菌后4 ℃保存备用。
2)体外成熟液(IVM):0.3 mmol/L胱氨酸+0.5 μmol/L半胱胺+1 μg/mL E2+0.5 IU/mL LH+0.5 IU/mL FSH+10% FCS+1% 双抗+0.22 mg/mL丙酮酸钠,用TCM199定容至50 mL,调节pH值为7.4,过滤灭菌后4 ℃保存备用。
3)10×精子洗涤液(10×SP-TL):4.675 0 g NaCl+0.230 0 g KCl+0.400 0 g NaH2PO4 +2.380 0 g HEPES,加双蒸水定容至100 mL。
4)90%梯度离心液(90% Percoll):16 mL 10×SP-TL+360 μL乳酸钠+0.336 0 g NaHCO3+144 mL Percoll+0.1 mol/L MgCl2+0.1 mol/L CaCl2,加双蒸水至160 mL,调节pH值为7.4,过滤灭菌后4 ℃保存备用。
5)SP-TALP(精子培养液):SP-TL基础液+6 mg/mL BSA(Frac V)+0.11 mg/mL丙酮酸钠+0.1%庆大霉素,0.22 μm过滤,4 ℃保存。
6)受精液(IVF-TALP):IVF-TL基础液+6 mg/mL BSA(EFAF)+100 μg/mL丙酮酸钠+0.1%庆大霉素,0.22 μm过滤,4 ℃保存。
7)胚胎培养发育液(SOF):0.017 2 g CaCl2·2H2O+0.009 97 g MgCl2·6H2O+0.016 2 g KH2PO4+0.053 37 g KCl+0.628 8 g NaCl+0.014 7 g Tri-Na-citrate+0.049 9 g肌醇+0.210 6 g NaHCO3+72.428 μL 乳酸钠+500 μL Gentamin+0.400 0 g EFAF BSA+1 000 μL丙氨酰-谷氨酰胺二肽+2 000 μL丙酮酸钠+1 000 μL 非必需氨基酸+2 000 μL 必需氨基酸,加双蒸水定容至100 mL,NaOH溶液调节pH值为7.4,过滤灭菌后4 ℃保存。
1.3 卵母细胞的收集与体外成熟培养
将捡卵液、成熟液(成熟液中分别添加100、200、400、800、1 600 nmol/L的AL-8810),在CO2培养箱预热2 h。选取卵巢表面直径为2~8 mm的卵泡,用10 mL注射器将卵母细胞吸出,转移至10 mL离心管中,静置沉淀15 min。用巴氏吸管弃掉卵泡液,导出底部沉淀,加入适量捡卵液稀释。在体视显微镜下用自制的口吸管捡出卵母细胞复合体(COCs),用成熟液洗涤3次。转移至体外成熟液中,于培养箱中培养22 h。统计第一极体排出情况。
1.4 免疫荧光染色法检测PTGFR的表达定位
将胚胎或卵母细胞在清洗液(含0.1% PVP的PBS缓冲液)中清洗3遍,每次2 min。转入4%多聚甲醛中固定30 min以上,移入TPBS缓冲液(含0.1%Tween2.0的PBS缓冲液)中清洗1 h,再进行透化1 h左右,透化液为含有0.5% TritonX-100的PBS缓冲液。用TPBS缓冲液清洗3次,进行抗原封闭,封闭液为添加1%BSA和0.1% Tween2.0的PBS缓冲液液,封闭完成后,移入一抗的工作液(1∶200)中4 ℃孵育过夜。用TPBS缓冲液清洗3次,移入二抗的工作液(1∶100)中37 ℃孵育。用TPBS缓冲液清洗3次后,用核染色剂(PI)避光染色10 min,用TPBS缓冲液清洗3次。固定到含有防荧光淬减剂的载玻片上,压片后在倒置荧光显微镜下观察染色情况。
1.5 实时荧光定量PCR检测PTGFR表达水平
根据QIAGEN RNeasy Plus Micro Kit操作说明分别提取卵母细胞、4-16细胞期胚胎、桑椹胚的微量RNA,反转录得到cDNA,再以反转录产物为模板进行qRT-PCR反应,反应体系为:cDNA 0.5 μL;4×QuantiTect SYBR Green PCR Master Mix* 5 μL;上下游引物各0.5 μL;RNase-free water 13.5 μL。反应条件为:95 ℃预变性3 min;94 ℃变性30 s,54.7 ℃退火30 s,72 ℃延伸30 s,35个循环;72 ℃延伸10 min。每个样本3个重复,RQ=2-ΔΔCt,以β-actin为内参基因,基因定量引物序列见文献[8]。
1.6 AnnexinV-FITC染色法检测抑制剂处理后卵母细胞凋亡
用AnnexinV-FITC细胞凋亡检测试剂盒进行卵母细胞早期凋亡检测。操作步骤如下:将COCs脱去颗粒细胞清洗干净,用盐酸(1 mol/L)去除透明带。取500 μL的Binding Buffer和5 μL的AnnexinV-FITC混匀后,再加入5 μL PI混匀,将混匀后的反应液加到96孔板的孔中,使每孔溶液量能覆盖住卵母细胞,将处理好的卵母细胞移入96孔反应液中,室温避光反应15 min,在荧光显微镜下观察、拍照,并统计数据。
1.7 体外受精与早期胚胎培养
取出冻精,37 ℃水浴解冻,剪开精细管,导出精子悬液,于常温离心机中离心10 min(2 200 r/min),弃上清。调整精子密度为1×107/mL,转移至获能液中孵育1 h。将成熟的COCs转移至受精液中,与获能后的精子进行共孵育,转移至培养箱中进行5 h的体外受精。受精完成后,捡出胚胎,脱掉多余的颗粒细胞,转移至胚胎发育液中进行体外培养(发育液中分别添加100、200、400、800、1 600 nmol/L的AL-8810),受精第2天计算卵裂率,受精第5天用SOF胚胎培养液洗涤3~5次,转入新制备的胚胎培养液(5% FCS)中继续培养。
1.8 统计分析
采用SPSS v18.0.0.统计软件进行统计分析。PTGFR基因表达水平数据采用单因素方差方法进行显著性分析,各组参与统计样本数为3;PTGFR抑制剂AL-8810对水牛卵母细胞凋亡、核成熟和早期胚胎的发育能力的的影响采用卡方(χ2)检验方法进行显著性分析,每组样本数>40,以P<0.05时为差异显著,P<0.01时为差异极显著。
2 结果与分析
2.1 PTGFR在水牛卵母细胞和胚胎中的表达定位
分别收集水牛卵母细胞与早期胚胎进行免疫荧光染色,结果如图1所示,PTGFR在MII期卵母细胞和4细胞期胚胎中均表达,在2个时期均存在PTGFR的染色体表达定位。
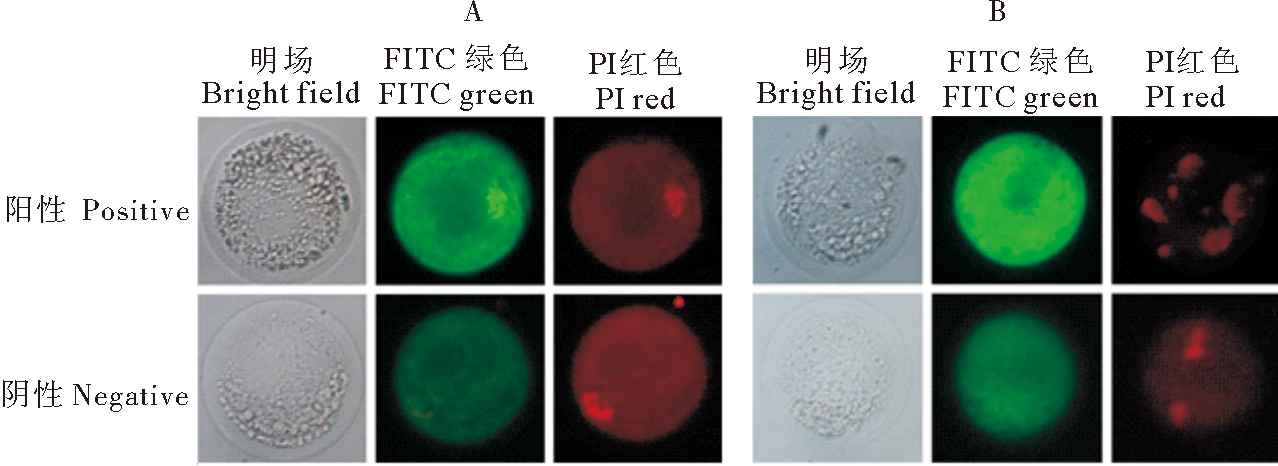
A :MII期卵母细胞 MII stage oocyte; B:4细胞期胚胎 4 cell stage embryo.
图1 PTGFR在水牛卵母细胞和胚胎中的表达定位
Fig.1 Expression localization of PTGFR in buffalo oocyte and embryo
2.2 PTGFR在水牛胚胎不同发育阶段的表达水平
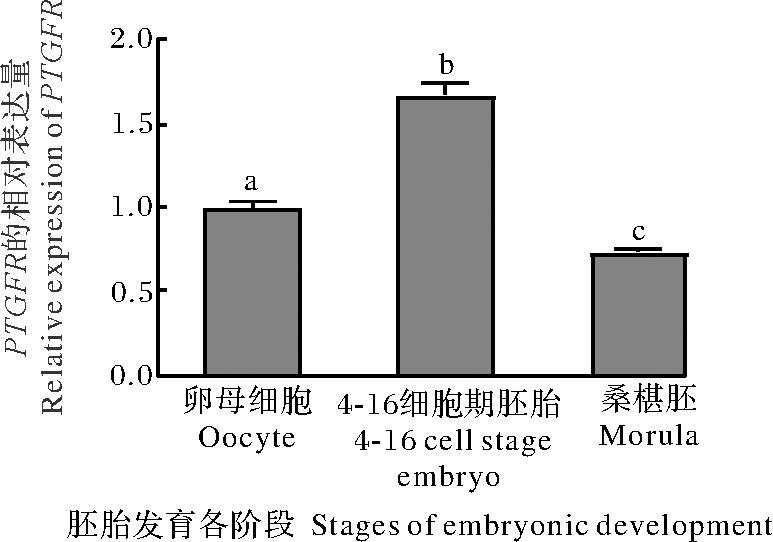
分别收集水牛卵母细胞、4-16细胞期胚胎、桑椹胚,实时荧光定量PCR方法检测PTGFR表达结果如图2所示。PTGFR表达水平在胚胎发育各阶段发生了明显的变化,在4-16细胞期胚胎中PTGFR表达量最高,在桑椹胚中表达量最低,且4-16细胞期胚胎中PTGFR表达量显著高于未受精卵母细胞和桑椹胚(P<0.05)。
2.3 AL-8810对水牛卵母细胞凋亡和核成熟效率的影响
收集卵母细胞复合体(COCs),用不同浓度(100、200、400、800、1 600 nmol/L)的AL-8810处理,Annexin V-FITC染色法检测MII期卵母细胞凋亡(图3A),结果显示各浓度处理组凋亡率均低于对照组,在AL-8810浓度分别为100、200和1 600 nmol/L处理组中凋亡率与对照组存在显著差异(P<0.05),高浓度(1 600 nmol/L)处理组可以显著降低水牛卵母细胞的早期凋亡发生率(图3B);以卵母细胞第一极体的排出作为核成熟标准,发现各浓度的AL-8810对水牛卵母细胞体外核成熟均无显著影响。
不同小写字母表示差异显著(P<0.05)。Different small letters mean significant difference (P<0.05) .
图2 PTGFR在水牛胚胎发育过程中的表达变化
Fig.2 Changes in expression of PTGFR from
buffalo embryos during developmental stage

A:凋亡示意图Schematic diagram of apoptosis;B:卵母细胞凋亡率,不同小写字母表示差异显著(P<0.05)。Apoptosis rate of oocytes,different small letters mean significant difference (P<0.05).
图3 AL-8810对水牛卵母细胞凋亡的影响
Fig.3 Effects of AL-8810 on apoptosis of buffalo oocytes
2.4 AL-8810对水牛IVF胚胎发育的作用
在胚胎发育液中添加不同浓度的AL-8810,检测其对早期胚胎发育效率的影响,结果显示,随着AL-8810处理浓度的升高8细胞期胚胎发育效率处于上升趋势,但无显著差异;在AL-8810浓度分别为100、200和800 nmol/L时,16细胞期胚胎发育效率显著高于对照组(P<0.05),且处理浓度为100 nmol/L时16细胞期胚胎发育效率高于处理浓度200 nmol/L和800 nmol/L的发育效率;在AL-8810浓度分别为100、400和800 nmol/L时桑椹胚发育效率大于对照组,但无显著差异。因此,在AL-8810浓度为800 nmol/L对水牛IVF胚胎发育效果最好。

不同小写字母表示组内差异显著(P<0.05)。Different small letters mean significant difference(P<0.05) within groups.
图4 AL-8810对水牛IVF胚胎发育能力的影响
Fig.4 Effects of AL-8810 on the developmental ability of buffalo IVF embryos
3 讨 论
卵母细胞的成熟是一个复杂的动态过程,受很多因素的影响,其成熟质量及效率对随后的胚胎发育能力有直接的影响。水牛的体外成熟培养体系中通常会添加激素、蛋白、能量物质及生长因子等来提高卵母细胞成熟质量[9]。特别是生殖激素对卵母细胞成熟、精卵结合以及囊胚发育都起着重要的促进作用,绝大多数卵母细胞成熟培养体系中都会添加各种生殖激素[10]。PGF2α是一种重要的生殖激素,其典型的生理功能包括溶解黄体、促进排卵、促进生殖道收缩等,但是高浓度的PGF2α会影响动物体内P4、E2、LH浓度[11-12],不仅对受精卵有直接的不利影响,而且可能引起黄体过早溶解,引发子宫提前收缩,从而导致妊娠失败,抑制PGF2α信号对改善卵母细胞成熟有非常重要的意义。本研究通过在水牛卵母细胞体外成熟液中添加不同浓度的PTGFR抑制剂AL-8810,发现抑制PGF2α信号虽然没有提高水牛卵母细胞核成熟效率,但显著抑制了卵母细胞的早期凋亡发生率。第一极体的排放是卵母细胞核成熟的标志之一,但不能作为反映卵母细胞质量的指标,部分卵母细胞尽管在体外成熟条件下能够排出第一极体,但并不具备受精或进行胚胎发育的能力[13]。卵母细胞只有在充分完成了核和胞质成熟才具备受精和支撑胚胎发育的能力[14],我们推测PTGFR抑制剂可能改善了水牛卵母细胞的胞质成熟。这也表明抑制PGF2α信号可以改善水牛卵母细胞成熟体系,使卵母细胞成熟质量得到提高,为体外胚胎生产等提供了有利条件[15]。
研究发现,循环血液中高浓度PGF2α会显著抑制桑椹胚发育至囊胚,奶牛妊娠率下降显著[16],奶牛胚胎移植的体外胚胎生产过程中用PGF2α受体抑制剂处理移植前胚胎,抑制了PGF2α对植入前胚胎发育的负面影响 [17-18]。但在水牛早期胚胎发育方面发挥的作用尚不明确,本试验系统研究了抑制PGF2α信号在水牛早期胚胎发育中的作用,揭示了PTGFR在水牛早期胚胎发育过程中的时空表达特性,说明PGF2α对卵母细胞和早期胚胎的抑制作用是直接的,这与在奶牛中的研究结果是一致的[19]。本研究结果显示,不同浓度的PTGFR抑制剂AL-8810对16细胞期胚胎发育效率有显著影响,对8细胞期胚胎和桑椹胚的发育效率也有提高,但差异不显著,可能PGF2α信号对胚胎的作用与本身受体的表达量有关 [20],囊胚阶段由于培养体系问题未能统计,在后续试验中会进一步优化培养体系去探究。
早期胚胎死亡率高是繁育面临的突出问题,牛在授精后前16 d胚胎损失率最高[21],妊娠早期子宫内环境中PGF2α浓度升高是造成早期胚胎死亡的原因之一,这是由于各种生理情况导致的,包括人工授精(AI)期间子宫的物理操纵等,发情配种后6 d内,已受精奶牛与未发情期相比PGF2α浓度增加了2倍以上[22-23]。虽然此时产生的PGF2α不会导致早期黄体的消退,但会直接影响胚胎的生长和存活[24]。此外,妊娠早期的P4水平对胚胎存活有着至关重要的作用[25],但是早期黄体用PGF2α处理后,会使P4浓度的上升延后,对妊娠造成不利影响[26]。因此,我们推测在授精前6 d用PGF2α受体抑制剂对牛进行治疗会提高胚胎成活率。这种活体水平实验需要较大的样本量、精准的PGF2α受体抑制剂使用剂量、以及严格的安全性评估。在后续研究中我们将在活体水平验证使用PGF2α受体抑制剂提高水牛繁殖力的可行性。
[1] 黄加祥,黄锋,诸葛莹,等.中国奶水牛产业发展综述——发展历程及趋势建议[J].中国奶牛,2019(12):1-8.HUANG J X,HUANG F,ZHU G Y,et al.Overview of the development of China’s dairy buffalo industry:development process and trend suggestions [J].China dairy cattle,2019(12):1-8(in Chinese).
[2] NEGLIA G,GASPARRINI B,BRIENZA V C,et al.Bovine and buffalo in vitro embryo production using oocytes derived from abattoir ovaries or collected by transvaginal follicle aspiration[J].Theriogenology,2003,59(5):1123-1130.
[3] DISKIN M G,WATERS S M,PARR M H,et al.Pregnancy losses in cattle:potential for improvement[J].Reproduction,fertility and development,2016,28(1/2):83-93.
[4] MAURER P R,BEIER H M.Uterine proteins and development in vitro of rabbit preimplantation embryos[J].Journal of reproduction fertility,1976,48(1):33-41.
[5] HINRICHS K,RIERA F L.Effect of administration of prostaglandin F2 alpha on embryo recovery from the uterus on day 5 after ovulation in mares[J].American journal of veterinary research,1990,51(3):451-453.
[6] ZHANG N,MAO W,ZHANG Y,et al.The prostaglandin E2 receptor PTGER2 and prostaglandin F2α receptor PTGFR mediate oviductal glycoprotein 1 expression in bovine oviductal epithelial cells[J].The journal of reproduction and development,2018,64(2):101-108.
[7] SCENNA F N,HOCKETT M E,TOWNS T M,et al.Influence of a prostaglandin synthesis inhibitor administered at embryo transfer on pregnancy rates of recipient cows[J].Prostaglandins & other lipid mediators,2005,78:38-45.
[8] 张姝.AL-8810对水牛卵母细胞体外成熟和早期胚胎发育作用的研究[D].武汉:华中农业大学,2013.ZHANG S.The role and regulatory mechanisim of AL-8810 on oocyte in vitro maturation and development of early embryo in buffalo [D].Wuhan:Huazhong Agricultural University,2013(in Chinese with English abstract).
[9] 贾振伟,田见晖,安磊,等.牛卵母细胞体外成熟技术研究进展[J].中国农业科学,2013,46(8):1716-1724.JIA Z W,TIAN J H,AN L,et al.In vitro maturation of bovine oocytes[J].China agriculture science,2013,46(8):1716-1724(in Chinese with English abstract).
[10] 丁爱军,周艳华,贾银海,等.生殖激素对奶水牛卵母细胞成熟及发育潜能的影响[J].中国奶牛,2008(11):18-21.DING A J,ZHOU Y H,JIA Y H,et al.Effects of reproductive hormones on oocyte maturation and developmental potential in milk buffalo [J].China dairy cattle,2008(11):18-21(in Chinese).
[11] GINTHER O J,FUENZALIDA M J,HANNAN M A.Pulsatility and interrelationships of 13,14-dihydro-15-keto-PGF2alpha(PGFM),luteinizing hormone,progesterone and estradiol in heifers[J].Biology of reprodution,2011a,84(5):922-932.
[12] GINTHER O J,HANNAN M A,BEG M A.Luteolysis and associated interrelationships among circulating PGF2α,progesterone,LH and estradiol in mares[J].Domestic animal endocrinology,2011b,41(4):174-184.
[13] HENDRIKSEN P J,VOS P L,STEENWEG W N,et al.Bovine follicular development and its effect on the in vitro competence of oocytes[J].Theriogenology,2000,53(1):11-20.
[14] CLAUDIA G P,LAURA D V,ANA L M,et al.Evaluation of zona pellucida birefringence intensity during in vitro maturation of oocytes from stimulated cycles[J/OL].Reproductive biology and endocrinology,2011,9(1):53[2020-03-31].https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3094282/.DOI:10.1186/1477-7827-9-53.
[15] 王娜,李姣,王小武,等.影响牛体外胚胎生产效率的因素[J].今日畜牧兽医,2018,34(11):1-4.WANG N,LI J,WANG X W,et al.Factors affecting in vitro embryo production efficiency of cattle[J].Today animal husbandry and veterinary medicine,2018,34(11):1-4(in Chinese).
[16] SEALS R C,LEMASTER J W,HOPKINS F M,et al.Effects of elevated concentrations of prostaglandin F2α on pregnancy rates in progestogen-supplemented cattle[J].Prostaglandins & other lipid mediators,1998,56:377-389.
[17] SCENNA F N,EDWARDS J L,SCHUENEMANN G M,et al.147 Pregnancy rates of recipient animals following application of a selective prostaglandin F2α receptor antagonist during embryo recovery[J/OL].Fertility,reproduction and development,2008,20(1):154[2020-03-31].https://www.publish.csiro.au/rd/RDv20n1Ab147.DOI:10.1071/RDv20n1Ab147.
[18] DEAVER S,FELIX A M,RHOADS M L.Reproductive performance of lactating dairy cattle after intrauterine administration of a prostaglandin F2α receptor antagonist 4 days after insemination[J].Theriogenology,2015,83(4):560-566.
[19] SCENNA F N,EDWARDS J L,PIGHETTI G M,et al.Presence of prostaglandin F2α receptor in in vitro-derived morula and blastocyst stage bovine embryos[J/OL].Reproduction,fertility and development,2006,18(2):180[2020-03-31].https://www.publish.csiro.au/RD/RDv18n2Ab143.DOI:10.1071/RDv18n2Ab143.
[20] GRYCMACHER K,BORUSZEWSKA D,SINDEREWICZ E,et al.Prostaglandin F2α(PGF2α) production possibility and its receptors expression in the early- and late-cleaved preimplantation bovine embryos[J/OL].BMC veterinary research,2019,15(1):203[2020-03-31].https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6570898/.DOI:10.1186/s12917-019-1939-0.
[21] DISKIN M G,MORRIS D G.Embryonic and early foetal losses in cattle and other ruminants[J].Reproduction in domestic animals,2008,43(2):260-267.
[22] KATARZYNA G,DOROTA B,EMILIA S,et al.Prostaglandin F2α(PGF2α) production possibility and its receptors expression in the early- and late-cleaved preimplantation bovine embryos[J].BMC veterinary research,2019,15(1):1-15.
[23] SCENNA F N,EDWARDS J L,ROHRBACH N R,et al.Detrimental effects of prostaglandin F2α on preimplantation bovine embryos[J].Prostaglandins & other lipid mediators,2004,73(3/4):215-226.
[24] SCHRICK F N,INSKEEP E K,BUTCHER R L.Pregnancy rates for embryos transferred from early postpartum beef cows into recipients with normal estrous cycles[J].Biology of reproduction,1993,49(3):617-621.
[25] LüTTGENAU J,KöGEL T,BOLLWEIN H,et al.Effects of GnRH or PGF2α in week 5 postpartum on the incidence of cystic ovarian follicles and persistent corpora lutea and on fertility parameters in dairy cows [J].Theriogenology,2016,85(5):904-913.
[26] CUERVO-ARANGOA J,GARCíA-ROSELLó E,GARCíA-MU OZ A,et al.The effect of a single high dose of PGF2α administered to dairy cattle 3.5 days after ovulation on luteal function,morphology,and follicular dynamics[J].Theriogenology,2011,76(9):1736-1743.
OZ A,et al.The effect of a single high dose of PGF2α administered to dairy cattle 3.5 days after ovulation on luteal function,morphology,and follicular dynamics[J].Theriogenology,2011,76(9):1736-1743.